A New Physician Payments Gold Rush
And probably lacking in transparency.
It is fairly well-established to track physician payments from pharma and traditional medical device manufacturers using the OpenPayments platform. It’s hardly perfect, and it probably doesn’t receive enough attention, but it’s still a solid effort.
But, is it well-suited to this new age of AI/ML platforms?
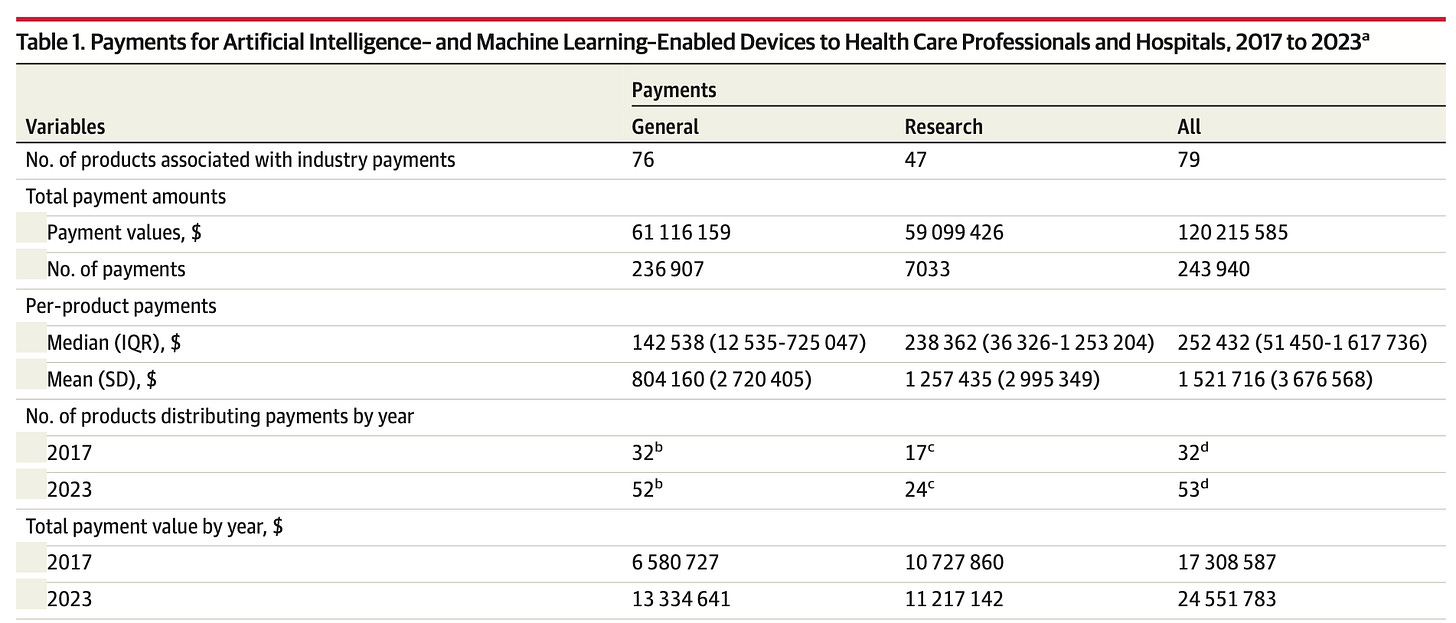
These authors checked the OpenPayments database for mention of any payments related to AI/ML products on the list of FDA-approved medical devices, and found a mere 79 out of the 846 listed devices were linked to physician payments:
Between 2017 and 2023, these payments totalled $61.1M in “general”(read: honoraria) payments and $59.1M in “research” payments, with a wide range of individual and institutional amounts.
The better question raised by the authors: is this just the tip of the iceberg?
Manufacturers of more than 90% of FDA-listed AI/ML–enabled devices publicly disclosed no payments to health care professionals or teaching hospitals from 2017 to 2023. This is likely a consequence of gaps in disclosure requirements, inconsistent compliance, and ambiguities in the regulatory classification of AI tools.
I suspect they’re absolutely correct about this – the consequence being further lack of transparency and potential biases affecting the evidence surrounding any validation for these products.