Another Baloxavir Advertorial In NEJM
Does anyone seriously make treatment decisions off this pharma junk?
Influenza season is dead, long live influenza season!
Baloxavir, known to most as Xofluza, is the newest of the influenza antivirals playing in heavy rotation these days. It’s not hardly new, having been around for 5-odd years, but there’s still room to sell more product – so, let’s publish more studies!
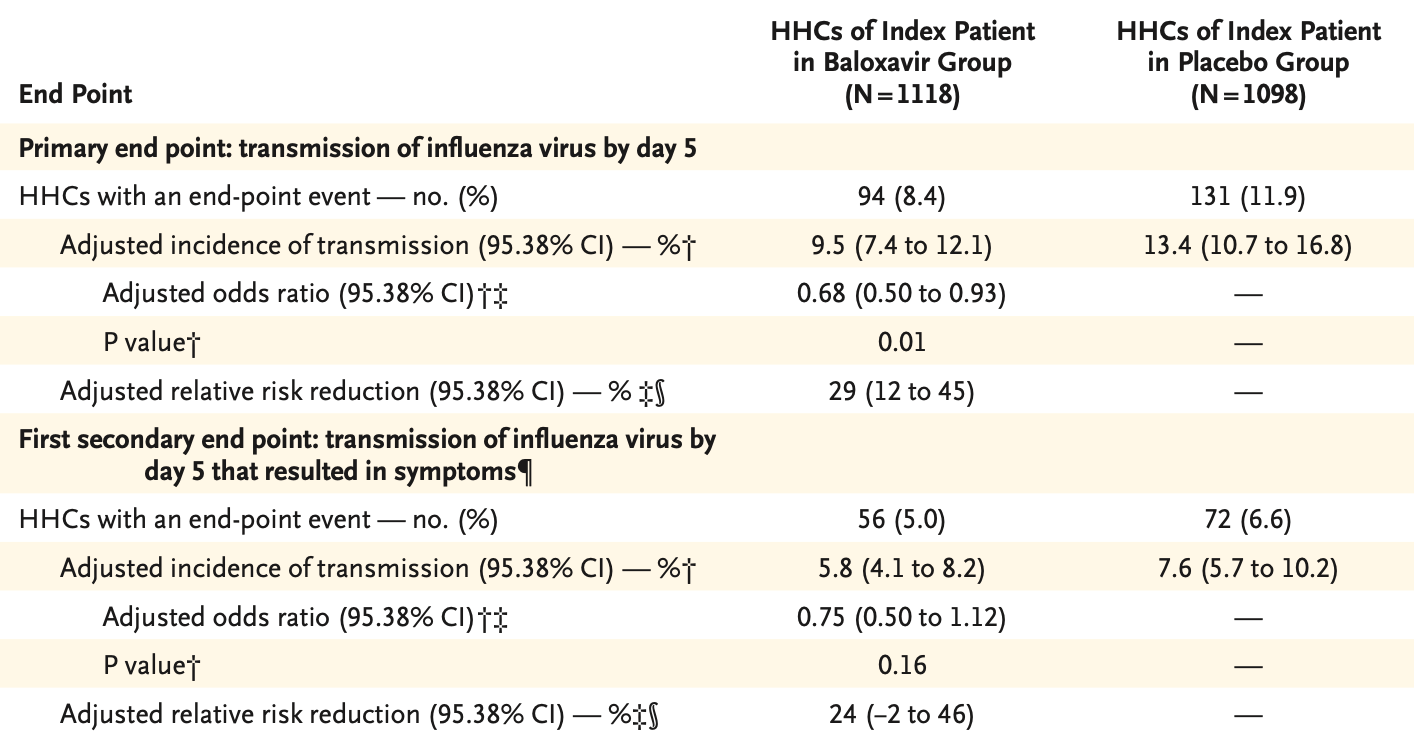
This is CENTERSTONE, the “let’s give baloxavir to folks and see if it prevents onward transmission to household contacts” trial – and the answer is: kind of?
As you can see, the overwhelming majority of household contacts of an index case of influenza never go on to develop symptoms, regardless of baloxavir use. The authors spend a great deal of time focusing on the relative risk reduction of ~30%, waxing poetic about how baloxavir could stop pandemic influenza in its tracks. Naturally, there’s a less-appealing calculus using the lens of absolute risk reduction and the number of courses of baloxavir required to stop one additional case of onward transmission – particularly at the ~$175 treatment course cost.
More exciting for pandemic influenza, the authors’ saw baloxavir induce mutations and substitutions in 7.2% of treated persons. This naturally raises concerns about baloxavir being more harm than good as a population- or pandemic-level treatment. Curiously, none of the household contacts of mutated influenza were found to have become infected with the mutated version, which the authors attribute to the onward transmission having occurred prior to baloxavir initiation. This illustrates how, even if baloxavir is effective at reducing transmission, an imagined use to prevent spread among household contacts may actually be mooted in a practical sense.
Finally, as with any trial conducted by, funded by, data interpreted by, and manuscript drafted by pharma and its medical writer cousins, every aspect of these data will be biased to show the product in the best possible light. Friends don’t let friends prescribe Xofluza.