COVID-19 Vaccines Safe in Pregnancy
More data unable to find fetal risks due to maternal exposure.
Every new treatment or intervention requires safety evaluation. Pre-approval clinical trials are critical steps, but will never fully detect rare adverse events, nor do clinical trials typically enroll all vulnerable populations. So, post-approval cohort studies and adverse event reporting systems remain important tools to ensure safety.
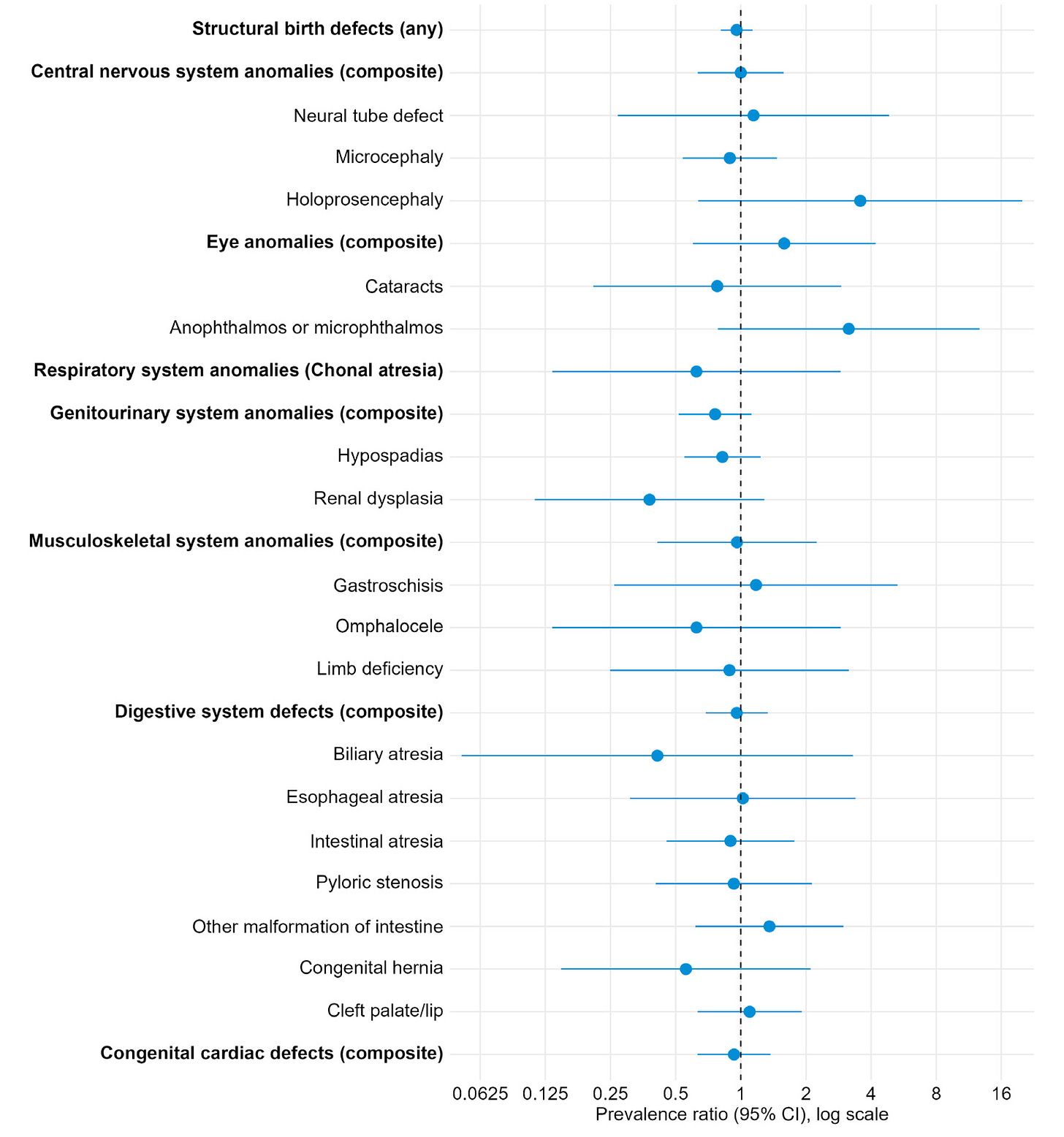
Here, we see a “claims-based” evaluation looking for diagnosis codes relating to structural birth defects associated with COVID-19 vaccination during pregnancy. This is the Merative Marketscan Commercial Database and Multi-state Medicaid claims database, so the analysis depends on the quality of these data and the completeness of the codes captured.
Regardless, as the lede above notes, no reliable signals of harm (nor protective benefit) were seen in children born to mothers having received either the Pfizer or Moderna vaccines:
It is, as always, worth noting a few investigators have COI with Pfizer and vaccine-related professional groups; the potential biasing influences of such must always be acknowledged. However, these observations showing no apparent risk are likewise consistent with similar findings from overseas.