Don't Bother With Head Injury Biomarkers
Despite the best efforts of BrainBox to sell them.
At some point, inevitably, various biomarker signatures relating to the breakdown of structural intracerebral proteins will be of value. That day has not yet come.
None of this prevents BrainBox + Abbott, however, from belaboring their GFAP + UCH-L1 product in the hopes of selling additional assays and incorporating its use into routine care.
In this study, the authors revisit TRACK-TBI, the initial traumatic brain injury study run from 2014 to 2018. This time, they reanalyze the data to look at whether its discriminatory capacity is modified by blood alcohol levels.
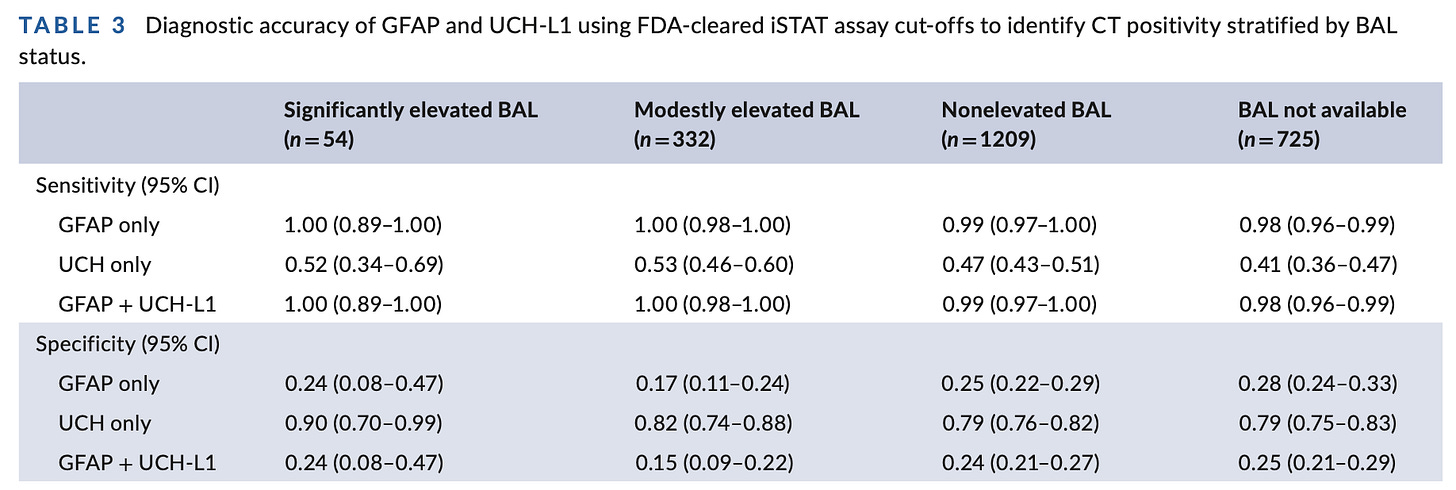
The key table:
Not too shabby – but, such small numbers the confidence intervals are quite wide “significantly elevated BAL”. Then, what is the acceptable miss rate for an intracranial injury, after all? Is it ~1%? A debatable point, but within the realm of consideration. And, I think that’s the point of these sponsored authors pushing these data out in a new publication.
However, TRACK-TBI is not ALERT-TBI. TRACK-TBI measured these biomarkers >9 hours from injury and sent frozen samples off to a central laboratory for analysis. ALERT-TBI implements these biomarkers in a practical fashion on the Abbott iStat device — and, to refresh your memory, sensitivity in ALERT-TBI was only 95.8% (95% confidence interval [CI] = 0.906 to 0.982). No should be signing up for an assay that misses ~1 in 20 intracranial hemorrhages – especially with its utterly dismal specificity.
Eventually, someone will get it right and the reality will live up to the promise – but that day is not today.