No Bacteria? No Antibiotics? No?
Changing practice is harder than providing a test result.
This is one of those lovely studies encapsulating the challenges facing the zillions of novel diagnostics companies in medicine.
Story: A company develops some sort of biomarker fingerprint and machine learning platform. The platform provides rapid results of apparent important clinical utility. Straight to market? Or try and prove the results affect clinical outcomes?
This study involves the “T2Bacteria panel”, a relatively-rapid whole blood test capable of detecting S. aureus, Enterococcus faecium, Klebsiella pneumoniae, P. aeruginosa, and Escherichia coli. In theory, detection or exclusion of these bacteria can support early narrowing of broad-spectrum anti-MRSA and anti-pseudomonal empiric coverage before blood culture results are finalized.
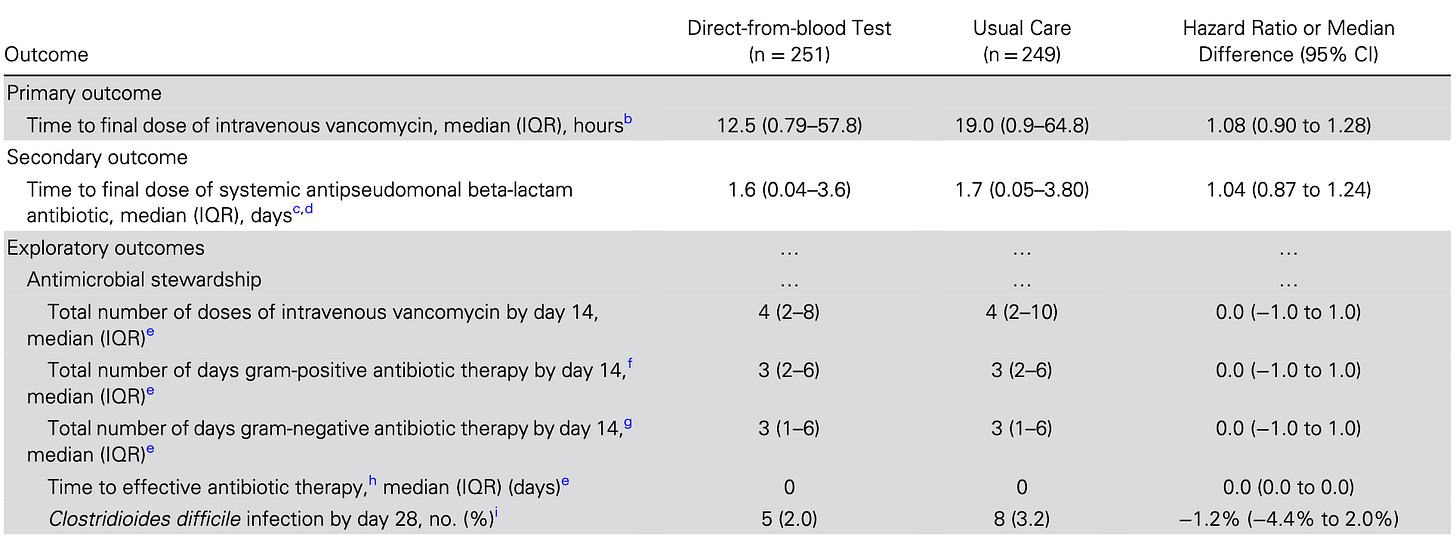
So, yes – results from T2Bacteria were available approximately 8 hours after randomization, about 10 hours sooner than the initial blood culture gram-stain, and a full five days sooner than the finalized blood culture result. And clinical practice shrugged:
And clinical outcomes were, likewise, no different.
And it also doesn’t help this company’s cause that performance was problematic – correctly identifying only 11 of this core set of 16 positive blood culture pathogens. Then, the number of false positives exceeded the number of true positives. It’s not going to support antibiotic stewardship to any practical extent if it is not possible to exclude the serious underlying condition requiring broad-spectrum coverage.
But, this shows the tension between sales (read: financial viability of a startup) and clinical medicine – moving the needle on clinical behavior is incredibly difficult, so it’s much easier to simply go to market based on surrogates and magical thinking.