No, Thrombolysis With DOACs Is Not Safer
Sometimes a "target trial emulation" is still just confounded observational data.
A lot of authors have piled into this latest clown car vehicle for the idea of administering thrombolysis to patients taking direct-acting oral anticoagulants.
There have been previous observational studies looking at this topic, but today these authors attempt to set themselves apart by calling this observational data a “target trial emulation”. A “target trial emulation” is meant to glean causal relationships from observational data by attempting to assign patients to two groups based on a premise of random assignment at time zero. This is appealing primarily because it’s cheap: you already have the data, and true trials are expensive.
This trial attempts to do the TTE thing for IVT in patients taking DOACs using ongoing stroke registries from 28 stroke centers and 1 national registry. In theory, to for a TTE to maintain the integrity akin to randomization from an RCT, patients meeting the defined inclusion criteria at time zero ought to be reasonably well-balanced. This would indicate clinicians were looking at these patients and making, based on true equipoise, an essentially random sort of eligible patients into different treatments.
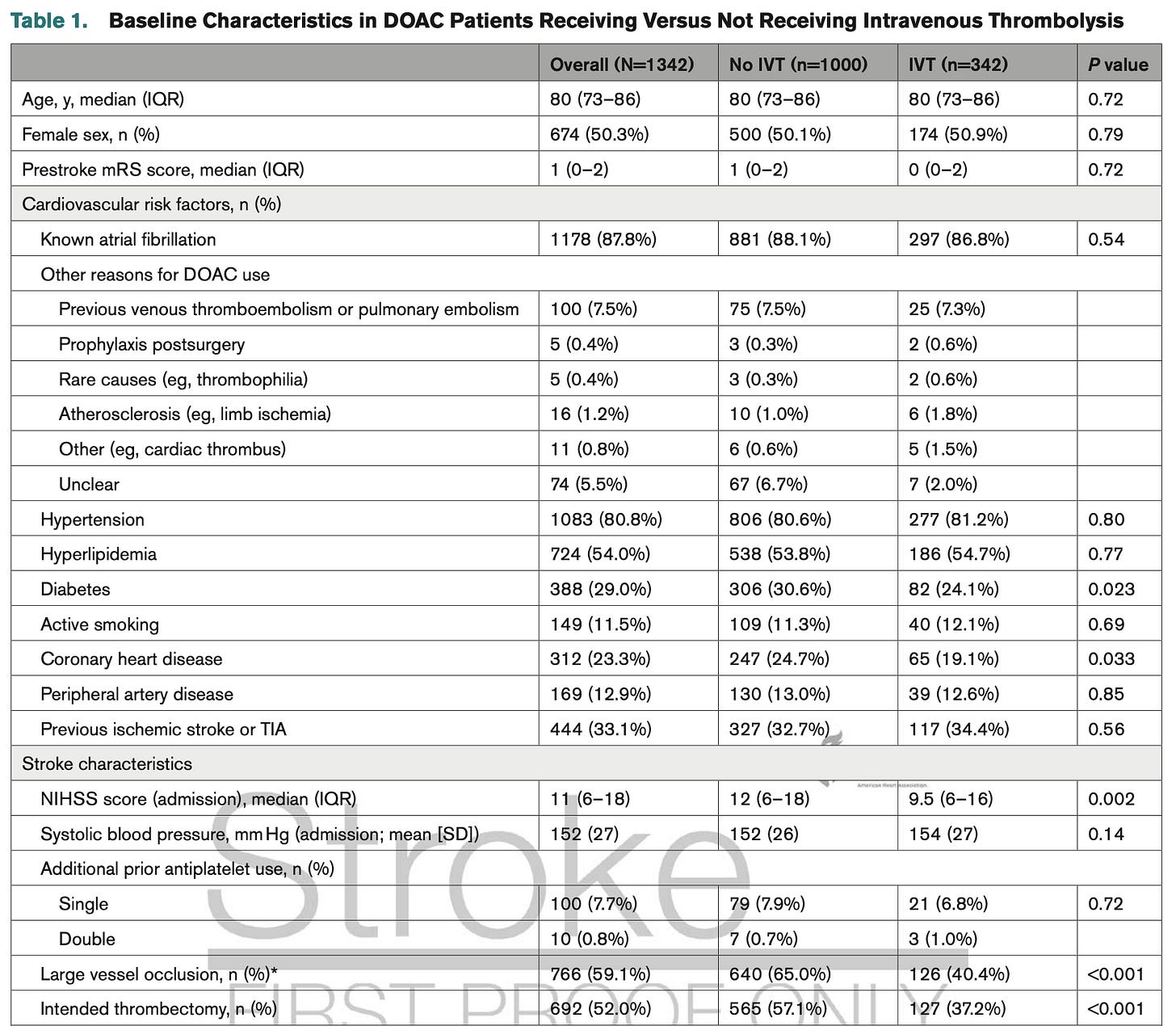
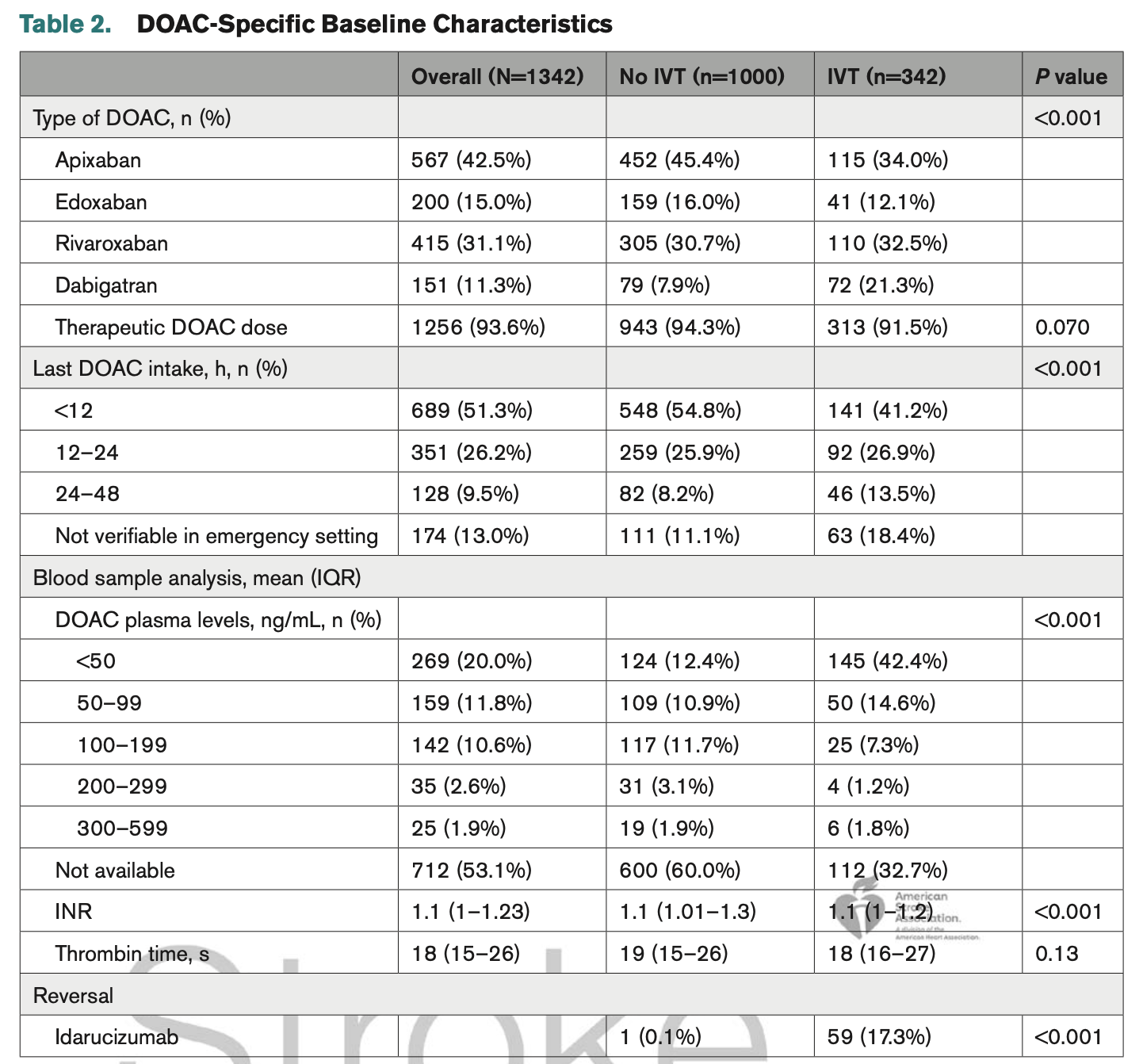
One quick look at the baseline characteristics here shows this is not the case:
Most prominent: different NIHSS, different “last known DOAC intake”, and, where known, different DOAC plasma levels. The patients receiving IVT are being chosen for IVT for reasons – and confounding by indication breaks the TTE.
This is just more junk observational data requiring adjustment, not nearly a TTE approximating an RCT. Thankfully, the authors do close by pointing to a need for a true RCT – two of which are already underway.