Paxlovid Spiralling Into Total Disutility
No one should be clamouring for this drug anymore.
There was great enthusiasm for the initial development of nirmatrelvir–ritonavir in the early days of the pandemic – a necessary tool, along with rapid roll-out of vaccines, in the grimmest weeks and months. However, the initial clinical trial enrolled primarily unvaccinated and previously-uninfected participants. By the time Paxlovid was widely available, vaccine uptake was quite widespread, leading to questions regarding its effect size.
Controversy ensued – and continues to ensue; what value remains, if any, for Paxlovid in our vaccinated world full of less-severe COVID-19 disease? One remaining play has been the idea it could be of use in “long COVID”: debilitating, persistent post-viral symptoms associated with prior COVID-19 infection.
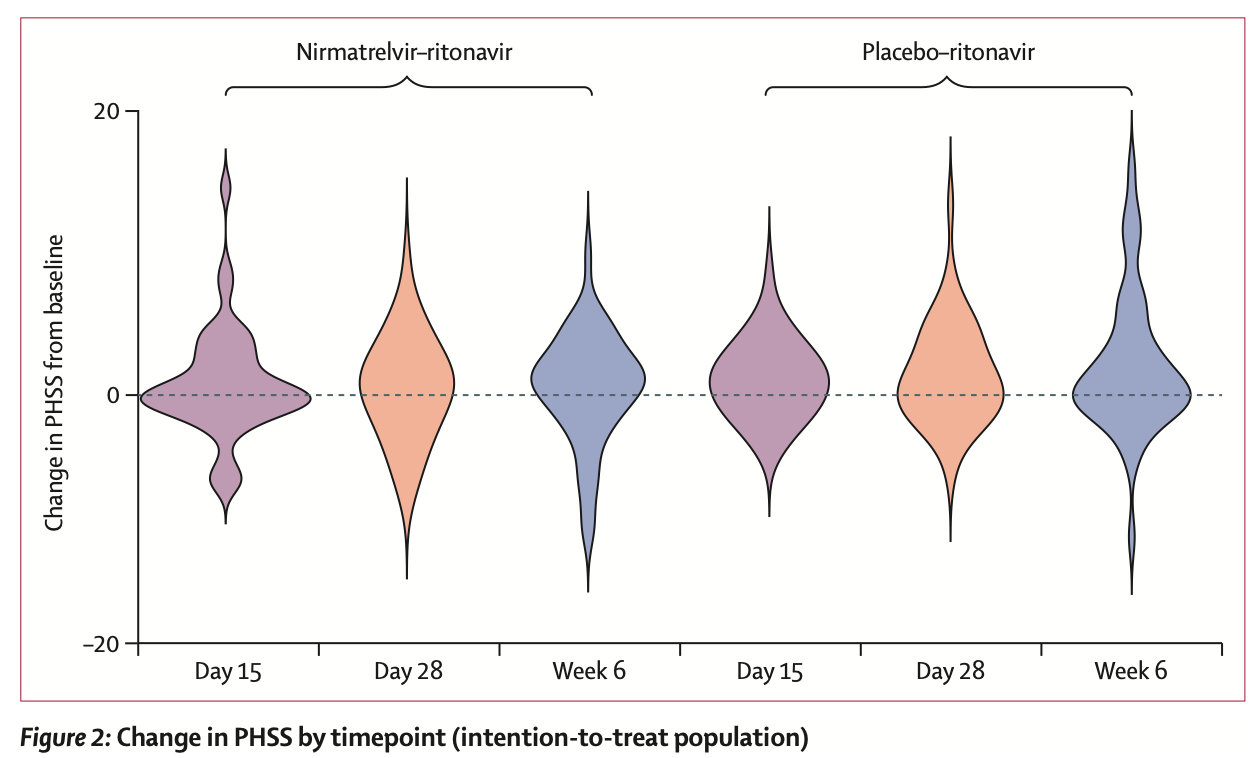
PAX LC is a small trial, with only 100 patients remaining in the mITT population by its conclusion, powered to detect a 5-point increase in the patient-reported “Physical Health Summary Score”.
The results are best summarized by this beautiful violin plot:
As you can readily see, there were no consistent beneficial effects to either Paxlovid or the “active” control, nor were there any differences between groups. This lack of effect was consistent across secondary outcomes except for safety – Paxlovid resulted in more drug-related treatment adverse effects, though none were severe and only a couple discontinued treatment. This matches the findings of STOP-PASC.
Friends don’t let friends take Paxlovid.