The Advantage of Experimenting On Pregnant Persons
Time to reconsider how human subjects protections are framed.
Pregnant persons are considered one of the vulnerable groups requiring special protections in medical research. This is obviously well-intended, as potential effects on fetuses require additional scrutiny.
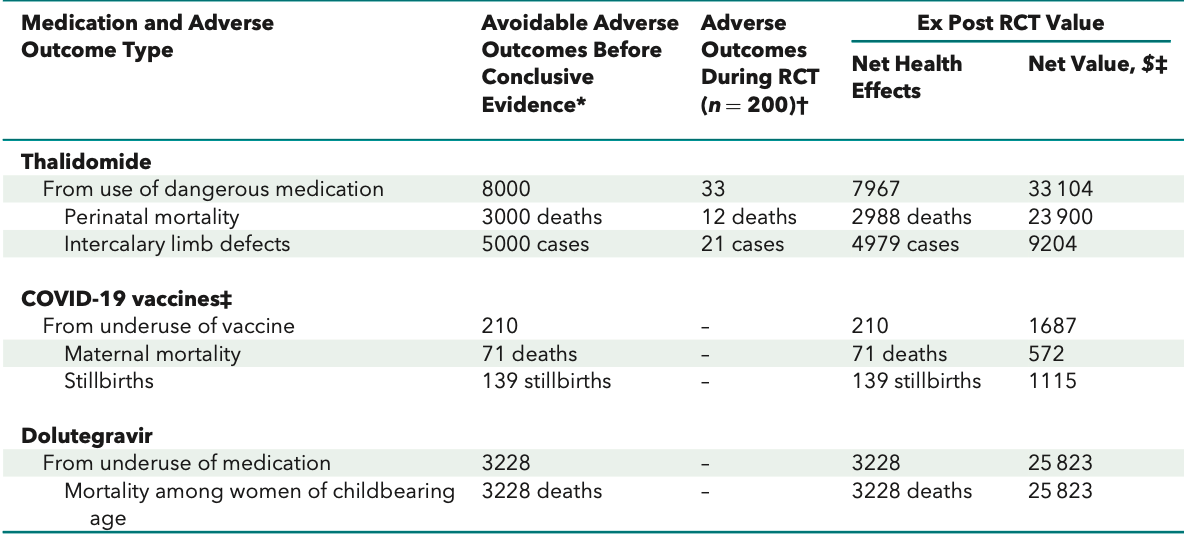
However, the net effect of this additional regulatory burden results in routine exclusion of pregnant persons from research. As this study highlights, this exclusion can result in far greater harms than would have been encountered during the conduct of research:
If, for example, pregnant persons had been included in trials evaluating thalidomide, the adverse outcomes seen in the study population may ultimately have prevented thousands of subsequent deaths. If pregnant persons had been included in trials of COVID-19 vaccines, the proven safety profile may have improved uptake during pregnancy, and prevented hundreds of deaths due to COVID during the peak waves. Finally, if pregnant persons had been prescribed dolutegravir as clinically appropriate, rather than less-effective alternatives, potentially thousands of deaths in women of child-bearing age may have been avoided.
These illustrative examples do not generalize to all research. However, when pregnant persons or persons of childbearing age are among the intended treatment population post-approval, the net result is passing a potential unknown risk along to women and fetuses. Where feasible and appropriate, regulatory bodies should consider mechanisms to encourage safe inclusion of pregnant persons in study populations.