The Blujepa EAGLEs Have Landed
Gepotidacin is on its way, but at what cost?
Starting out with my standard line on this topic: A new antibiotic! Let’s try to never use it.
These trials – EAGLE-1, -2, -3 – are all focusing on the clinical utility of gepotidacin, a triazaacenaphthylene inhibiting bacterial DNA gyrase and topoisomerase IV. This is a novel target, potentially sidestepping some of the other bacterial resistance mechanisms affecting fluoroquinolone and trimethoprim‑sulfamethoxazole efficacy.
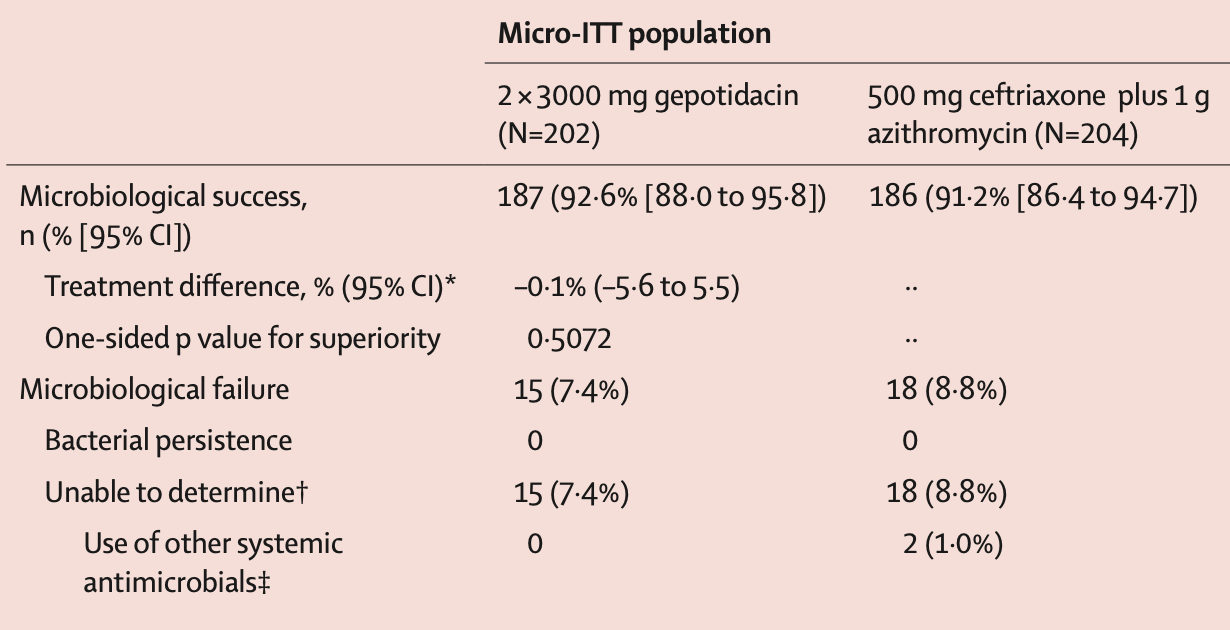
EAGLE-1 is the “new” publication this week, and this is the non-inferiority comparison against ceftriaxone plus azithromycin. Most of the N. gonorrhea isolates expressed some resistance to current antibiotic options. Their topline effectiveness results are here:
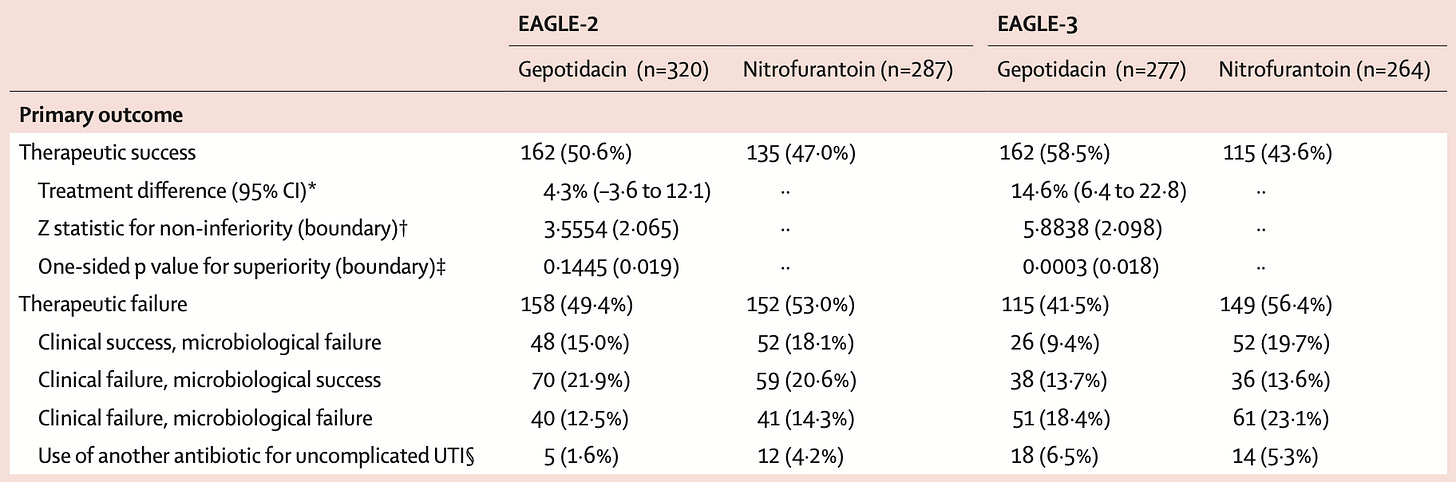
And, then, EAGLE-2 and -3 are the trials evaluating its use for uncomplicated cystitis:
As with all trials, there are always exclusion criteria potentially relevant and likely to diminish its real-world effectiveness. Then, among these few hundred study participants, there were few safety signals, but diarrhea – including episodes of C. difficile – were more prevalent with gepotidacin. This is obviously too small a sample to identify rare serious events, which will require ongoing post-approval monitoring. Lastly, these trials all suffer from the heavy influence of GlaxoSmithKline, and ought be interpreted through that lens.
But, gepotidacin appears to have a promising role – applied narrowly to as few patients as possible to prevent induction of new resistance, for use in those situations where treatment failure with conventional options is of greater concern than the unknown risks associated with this new antibiotic. We also do not yet know its cost – pending a willingness-to-pay market evaluation, no doubt.