The RSV Vaccine Worked in Scotland
Some countries are trying to move forward instead of backward.
This brief report examines the rollout in Scotland of the Pfizer Abrysvo vaccine for respiratory syncytial virus.
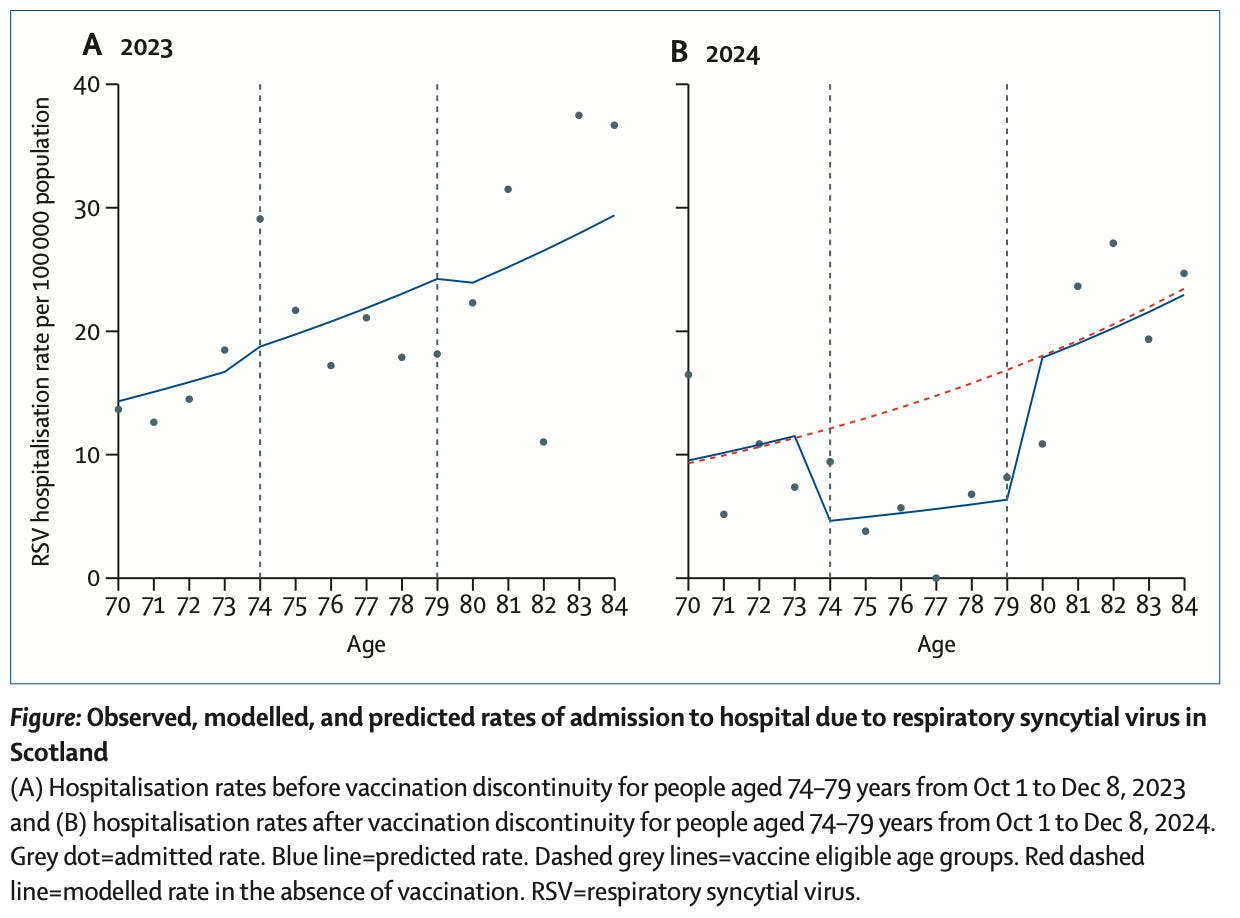
Typically, in Scotland, annual hospitalization rates for the elderly are in the 20-30 per 100,000 population aged 74 and older. Following approval of the RSV vaccine in the UK, Scotland undertook a pilot vaccination program targeting adults aged 75-79 years – and managed an impressive uptake of 201,891 out of 294,506 by November of 2024. They then compared seasonal hospitalization rates in this cohort to both the prior year, 2023, and to an “expected” hospitalization rate based on the burden of hospitalization seen in those aged 70-73 and 80-84.
Here are the main results:
Hospitalizations, roughly speaking, were a little more than halved in the age group targeted by the vaccine.
So, yes – it works. It’s also unclear how cost-effective it is at current pricing. Wholesale cost in the U.S. is roughly $300 – and, although bulk contracts likely receive discounted pricing, the cost of wide vaccination in Scotland still likely ran into the tens of millions of dollars to save fewer than 100 hospitalizations. There are other downstream health burdens and costs following hospitalization for which to account, but work need yet be done to best utilize the new RSV vaccines.