The SABA+Inhaled Corticosteroid Superiority
Otherwise, I hate everything about this trial.
Today’s trial is BATURA, the follow-up to MANDALA. These trials are trying to put the kibosh on SABA monotherapy, as can be seen in GINA.
But, as the authors say, SABA+inhaled corticosteroid still lacks the FDA-approved indication for mild asthma. Does BATURA mimic MANDALA?
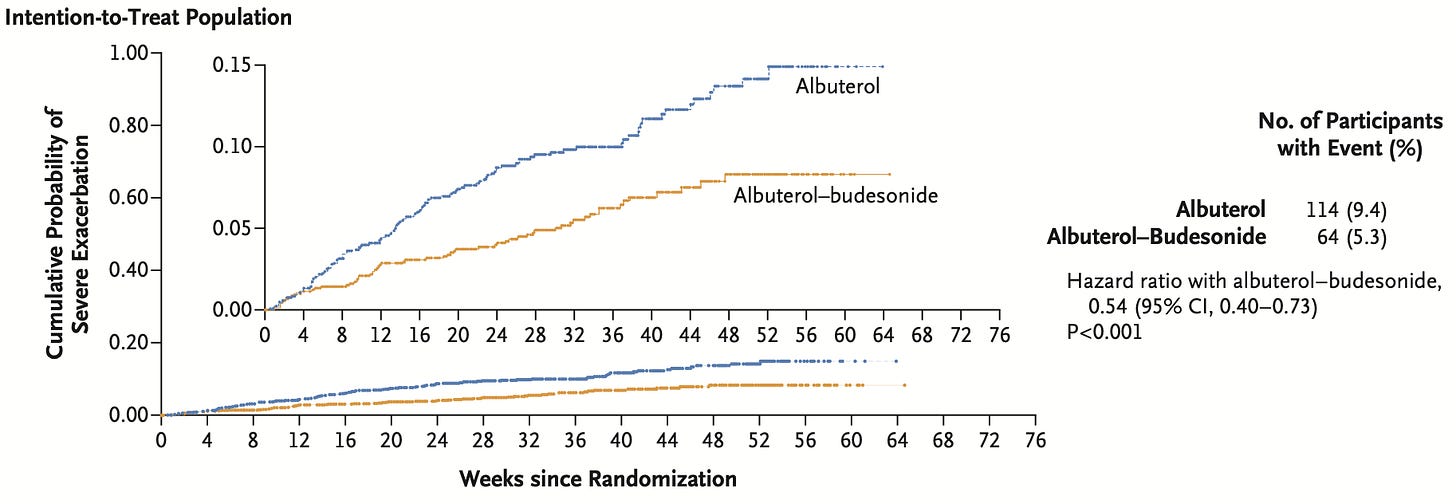
Yep:
By all means, quibble with whether the participants were on appropriate maintenance therapy at baseline, considering the extent of their daily-ish rescue inhaler use. Come along as I rage at the pervasive influence of AstraZeneca in designing and orchestrating this trial. Note that 20% of the trial population was lost to follow-up.
But, there were fewer “severe” exacerbations and a reduced per-participant exposure to systemic corticosteroids. The “number needed to treat” is relatively high, but the SABA+inhaled corticosteroid probably still wins the economic game preventing lost days of work and recovery. And, finally, it just makes sense – SABAs are a one-trick pony that don’t address the underlying inflammatory state triggering the bronchospasm.
If you’re still using SABA monotherapy for anything in the outpatient arena, it’s probably not the best option for your patients.