TriVerity: A More Expensive Procalcitonin
... which, itself, is a more expensive CRP.
There’s always breathless excitement about a new sepsis diagnostic test. Everyone ought to remember the heyday of procalcitonin a few years back, and the literature saturation achieved from trials sponsored by Thermo Fisher, Roche, et al.
Now we have TriVerity, another entry into the field. The new validation data about TriVerity has actually been around, in some form or another, since at least January. At that point, it was possible to glean some data from preprints and the FDA approval of their testing platform. Our friends over at PulmCCM took a look at some of this early data.
Inflammatix is back in the news, however, because they’ve pushed three open-access publications into Nature. The most important of these, or, at least the one that will help push product, is their peer-reviewed publication of SEPSIS-SHIELD. This is their main validation vehicle, in which patients with suspected infection and an abnormal vital sign had a blood sample taken, their outcomes clinically adjudicated as bacterial or viral (or other), and outcomes were compared with their TriVerity scores:
Pretty slick looking, eh? It’s a benchtop QT-LAMP device that amplifies 29 mRNA components looking for gene expression associated with infection. This means, despite all its technological wizardry, it’s still doing basically the same thing as all the other “sepsis diagnosis tools” – looking to the body’s own immune system activation as a surrogate for the bacteria or virus wreaking havoc.
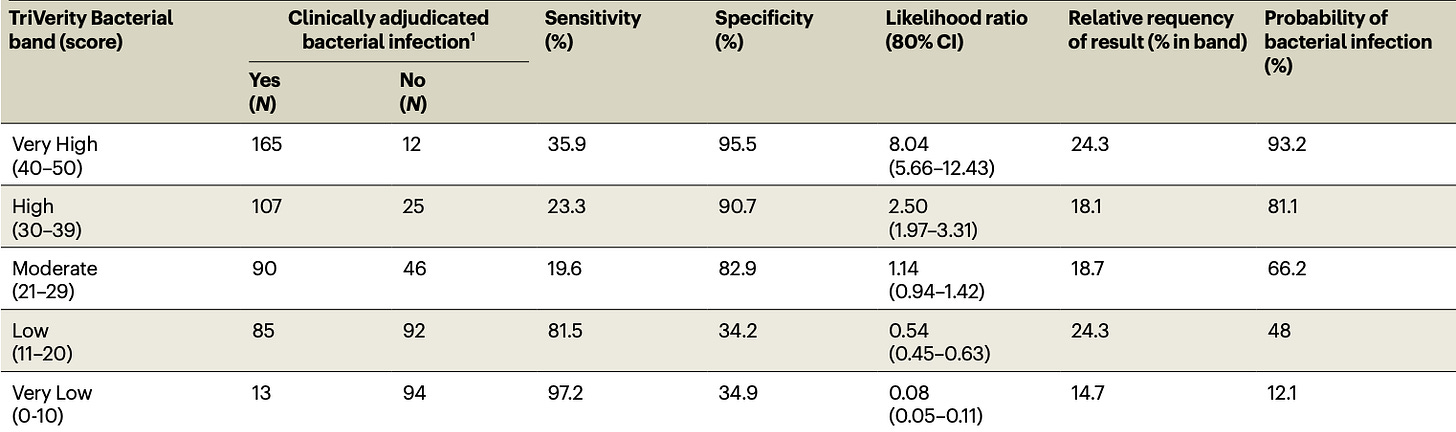
They claim it’s pretty good at what we care about – identifying a bacterial infection:
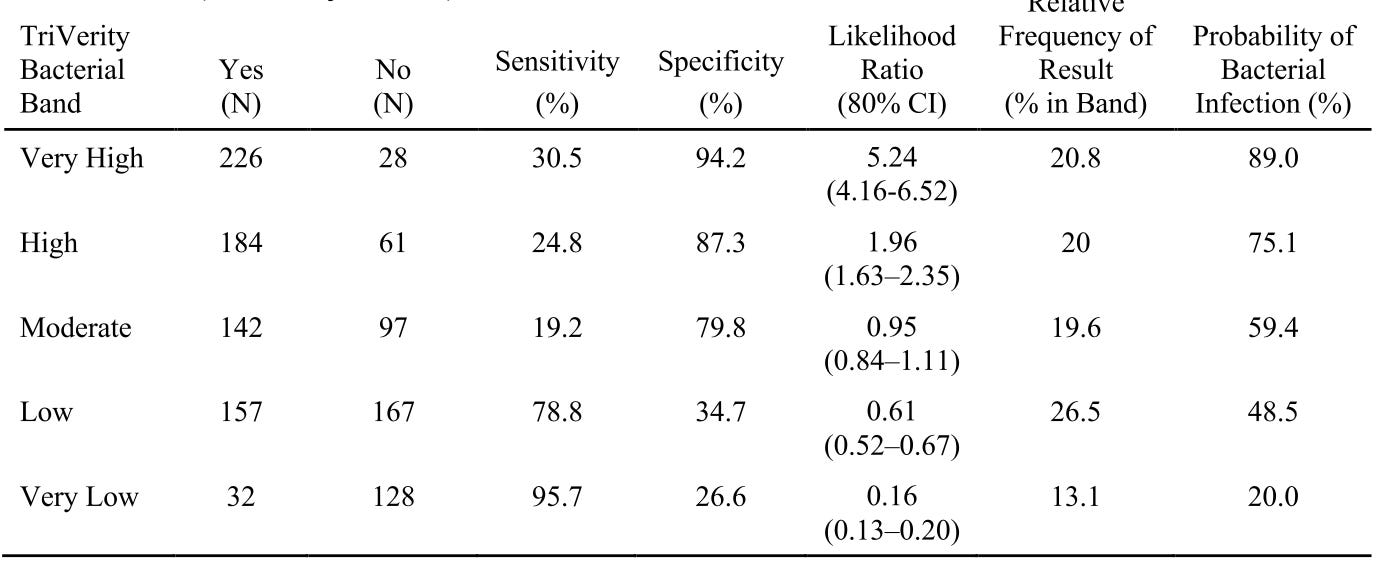
However, this is based off the 700-odd patients for which there was “clinical consensus”, as adjudicated by their methods described here. Tucked into their extended data tables are the performance characteristics you should care about, where the actual clinical uncertainty of daily life abounds:
We’re regressing back towards CRP and procalcitonin territory, here – and those haven’t exactly revolutionized early care for sepsis or aided substantially in antibiotic stewardship.
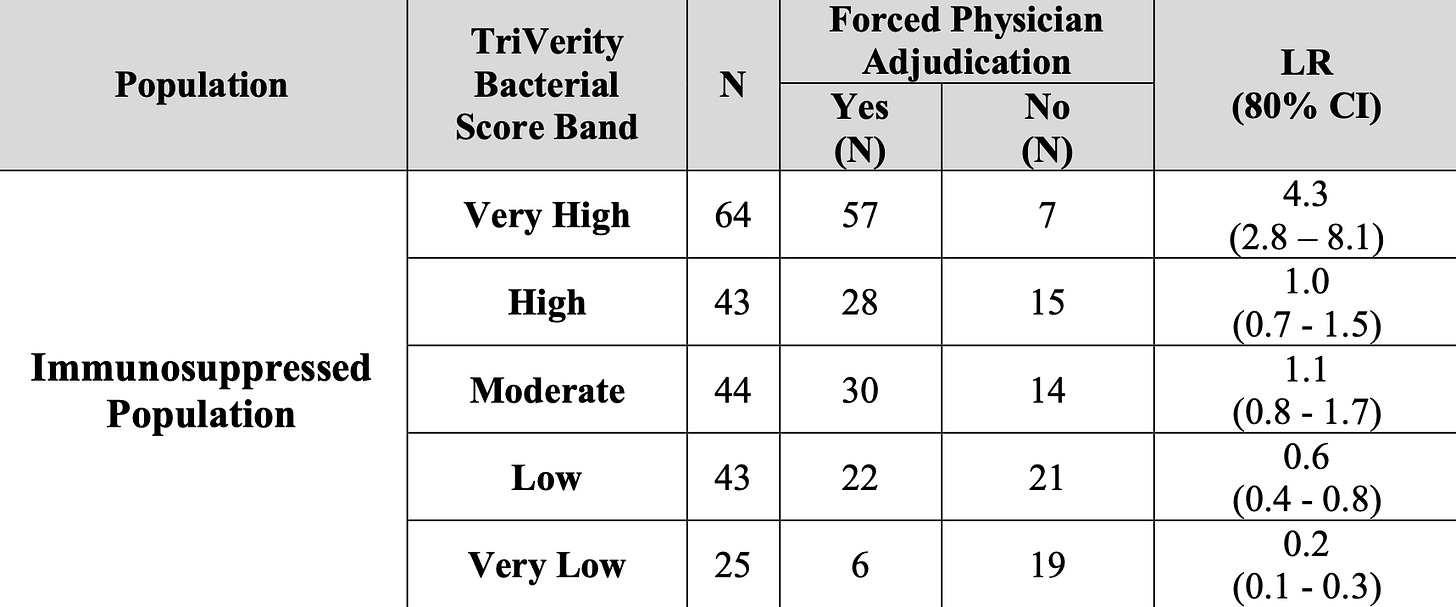
Lastly, there are still some big holes in SEPSIS-SHIELD of ongoing concern. They report enrolling patients with “immunosuppression”, but that category was dominated by the presence of solid malignancy. This doesn’t accurately capture whether they were undergoing active cytotoxic therapies, and other, true immunosuppression (transplants, steroids, etc.) were a tiny fraction of patients. Performance reported in the FDA document is not confidence-inspiring:
Lastly, the median age of included patients was only 52 (IQR 36-64). The patients who are 1) the worst about overtly expressing their infection, and 2) some of the highest-risk for poor outcomes, are the elderly. This study does not enroll nearly enough older persons to be generalizable for use in this cohort. A reliable test would be a treasure if it could properly classify the causes of delirium in the elderly as bacterial, viral, or non-infectious – and this ain’t it.
Color me unimpressed – and that’s just this bland diagnostic performance data, not a useful look at how this information impacts clinicians, changes behavior, or (gasp) improves patient outcomes …